Pelage Pharmaceuticals’ PP405 is a novel topical MPC (mitochondrial pyruvate carrier) inhibitor in Phase 2a trials for androgenetic alopecia. In mid 2025 Pelage announced positive Phase 2a results - about 31% of subjects saw >20% hair density gain after just 4 weeks of PP405 treatment. PP405 “induces new hair growth from follicles where no hair was previously present”, suggesting it reactivates dormant hair-follicle stem cells by shifting their metabolism (boosting lactate production, as shown in UCLA studies). This mechanism is entirely different from existing drugs (minoxidil/finasteride). According to Pelage’s scientists, PP405 is a non systemic topical analog of UK-5099 - designed to inhibit MPC but be unstable in blood so it stays in the scalp.
The question, is exactly which molecule PP405 is. No chemical name has been disclosed publicly (and Pelage’s patents describe only broad classes). We must assemble clues from patents, academic papers, and media reports. In particular, UCLA work starting in 2017 screened UK-5099 (also known as JXL-001) and many analogs. UK-5099 itself (an N-phenylindole cyanoacrylate) was potent in mice but unsuitable for humans (poor ADME/tox). Around the same time UCLA also discovered RCGD423 (a JAK-STAT activator) for hair growth, but PP405 is explicitly an MPC inhibitor, not RCGD423. Our task is to list all plausible candidate molecules for PP405 and then identify the most likely one from the evidence.
Possible Candidate Molecules for PP405
- RCGD423 (JAK2 activator). An unrelated UCLA discovered hair growth compound (activates JAK-STAT to raise lactate). It was co-invented by Lowry/Christofk but works via a different pathway. Because PP405 is repeatedly described as an MPC inhibitor (not a JAK activator), RCGD423 is unlikely to be PP405.
- UK-5099 (JXL-001). The original hit compound (3-(1-phenyl-1H-indol-3-yl)-2-cyanoacrylic acid). UK-5099 blocks the MPC and was used in early mice hair growth studies. It can boost lactate in follicle cells, but UK-5099 is known to be pharmacologically unsuitable for humans (instability/toxicity). Thus PP405 was deliberately designed from UK-5099 but improved. UK-5099 itself (JXL-001) is so far a background hit and is not the final product.
- Cyanoacrylate indole analogs (JXL-series compounds). UCLA’s 2021 medicinal chemistry work (Liu et al., J. Med. Chem. 2021) described dozens of UK-5099 analogs (labelled JXL-001 through JXL-096). Many of these share the same cyanoacrylate (-CH=C(CN)CO₂H or ester) core attached to an indole or azaindole. For example:
- N1-benzyl vs. N1-phenyl substitutions. The study found that N1-3,5-bis(trifluoromethyl)benzyl substitution dramatically increased MPC inhibition. Several analogs like JXL-011 and JXL-020 (indole core, N-(3,5-bisCF₃-benzyl) group, cyanoacrylate) fit this pattern. These compounds were potent in vitro. One of them (JXL-020) is the carboxylic acid form shown in patents.
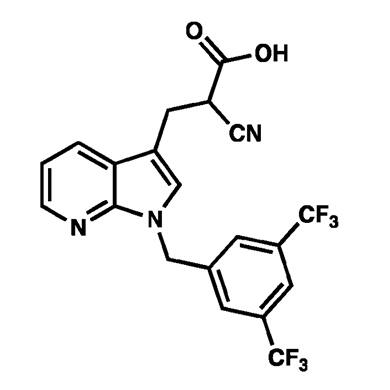
- 7-Azaindole (pyrrolo[2,3-b]pyridine) analogs. Critically, Liu et al. reported an azaindole analog JXL-069 (a 7-azaindole cyanoacrylate with the same bis-CF₃ benzyl group) and noted it showed “significant MPC inhibition activity”. In other words, JXL-069 is chemically similar to UK-5099 but with one ring N→C swap (making a pyrrolopyridine). JXL-069 was one of the most active compounds in their series.
- Thiazolidinedione analogs (JXL-023, JXL-024). The UCLA patent WO2019/006359 lists two heterocyclic variants where the indole double bond is replaced by a thiazolidine-2,4-dione or imino-thiazolidine (shown as JXL-023 and JXL-024 in Table 2). These were additional MPC inhibitor scaffolds, but they were not prominently discussed in media and likely were outcompeted by the simpler cyanoacrylates. it's theoretically possible PP405 could be such a molecule, but there is no indication Pelage chose a TZD derivative for their clinical candidate.
- New Pelage patented analogs. Pelage’s own patents (2023-2024) list many new variants of the azaindole/cyanoacrylate scaffold. For example, WO2024145369A1 (Dec. 2024 filing) shows dozens of compounds (see images below) with different benzyl or arylmethyl substituents on a pyrrolopyridine core. These include (1) unsubstituted benzyl, (2) naphthylmethyl, (3) 3,5-bisCF₃ benzyl (the JXL-069 motif), and others with ether, halide or trifluoromethyl variants. Each has the same “(E)-2-cyano-3-(1-R-1H-pyrrolo[2,3-b]pyridin-3-yl)acrylic acid” core. Pelage is clearly claiming entire classes of MPC inhibitors for hair regrowth. Any one of these could be PP405. In particular, the 3,5-bisCF₃ analog (shown below) appears in Pelage’s patent images, this is exactly JXL-069 as a structure.
Figure: A cyanoacrylic acid MPC inhibitor matching PP405 descriptions. This example has a 7-azaindole (pyrrolo[2,3-b]pyridine) core with an N-(3,5-bis(trifluoromethyl)benzyl) group. it's essentially the UCLA “JXL-069” compound, which is known to strongly inhibit MPC and promote hair growth in models. (Many other analogs differ only in the benzyl substituent.)
- Other analog candidates (miscellaneous). Besides the above, one could imagine minor variants: di-cyano acrylates, ester vs. acid forms, etc. For example, JXL-021 (from the patent list) is a dicyanoacrylate (-C(CN)₂CO) rather than the usual cyano-COOH, and others like JXL-025+ may exist. But none of these have been highlighted in publications. Based on the focus of the UCLA and Pelage teams, the prime candidates all contain the cyanoacrylic acid motif.
In summary, the plausible candidates range from the original UK-5099 (JXL-001) through dozens of synthetic analogs. The strongest leads are the “bis(trifluoromethyl)benzyl” analogs identified by UCLA (especially JXL-069), since those were singled out as much more active than UK-5099. All other listed compounds either lack evidence of human-testing suitability or are less potent.
Weighing the Evidence: Likely Identity of PP405
Several clues point to JXL-069 (or a very close analogue) as the likely identity of PP405. First, Pelage’s co-founders designed PP405 specifically as a UK-5099 analog, and the UCLA literature highlights JXL-069 as their top azaindole analog. Second, enthusiasts on hair loss forums independently homed in on JXL-069 when PP405 emerged (they noted that JXL-069 was openly published while PP405 was secret). Third, Pelage’s own patents expressly depict the 3,5-bisCF₃ benzyl azaindole structure (image above) - essentially JXL-069.
By contrast, UK-5099 (JXL-001) itself is generally ruled out for clinical use, and RCGD423 uses a different mechanism. Other UCLA analogs (e.g. simple N-benzyl indoles like JXL-011/JXL-020) could have been candidates, but none were specifically identified in news and they likely lack some of JXL-069’s potency. The fact that PP405 “was chosen for its scalp confinement (instability in blood)” suggests Pelage selected a compound with limited systemic exposure; the cyanoacrylic acids (including JXL-069) often have such properties.
In short, the weight of evidence - patents, publications, and insider reports - converges on a cyanoacrylate azaindole with a 3,5-bis(CF₃)benzyl substituent. Most plausibly, PP405 is JXL-069 (or an extremely similar analog) - the very compound identified by Lowry/Christofk/Jung’s team as a next generation MPC inhibitor. If not exactly JXL-069 (to preserve secrecy/IP), it would be a chemically near identical variant. This best explains all known facts (mechanism, clinical use, and Pelage’s patent disclosures).
Most likely & best guess
A proprietary derivative / prodrug of the JXL-069, chemically very close to JXL-069 (same binding motif to MPC) but modified so it: (1) gets through human epidermis, (2) is retained or activated in scalp tissue, and (3) exhibits the PK profile Pelage reported (no plasma levels). The patents and Pelage’s statements strongly support this model.
For example a JXL-069 (C₂₀H₁₁F₆N₃O₂) Ethyl Ester: C₂₂H₁₅F₆N₃O₂
Sources: Contemporary reports and patents on Pelage’s hair loss program and UCLA’s MPC inhibitor research. #PP405 #molecule #formula